

Table 9.1.1 shows that LiCl is soluble in water, but BaSO 4 is not soluble in water.Īlthough soluble barium salts are toxic, BaSO 4 is so insoluble that it can be used to diagnose stomach and intestinal problems without being absorbed into tissues. Once the chemical identity of the solid product is determined, we can then determine the balanced formula equation, the complete ionic equation as well as the net ionic equation, describing the chemistry that has occurred. Refer to the solubility rules table to determine insoluble products which will therefore form a precipitate. In this case, it is AgCl which is the precipitate. No need to correct the formula as both compounds already have their charges balanced. The only possible exchange reaction is to form LiCl and BaSO 4.Ĭorrect the formulas of the products based on the charges of the ions. Mixing the two solutions initially gives an aqueous solution that contains Ba 2 +, Cl −, Li +, and SO 4 2− ions. Predict what will happen when aqueous solutions of barium chloride and lithium sulfate are mixed.Ĭhange the partners of the anions and cations on the reactant side to form new compounds (products).īecause barium chloride and lithium sulfate are strong electrolytes, each dissociates completely in water to give a solution that contains the constituent anions and cations.
As stated previously, if none of the species in the solution reacts then no net reaction occurred. (Water molecules are omitted from molecular views of the solutions for clarity.)Ī precipitation reaction occurs when a solid precipitate forms after mixing two strong electrolyte solutions. The reaction occurs when oppositely charged ions in solution overcome their attraction for water and bind to each other, forming a precipitate that separates out from the solution. Because no net reaction occurs, the only effect is to dilute each solution with the other. In a precipitation reaction, aqueous solutions of soluble salts react to give an insoluble ionic compound the precipitate. Figure 9.2.1: The Effect of Mixing Aqueous KBr and NaCl Solutions. When these solutions are mixed, the only effect is to dilute each solution with the other. As you will see in (Figure 9.2.1), none of these species reacts with any of the others.

Injected chemicals may also be incompatible with each other or other constituents within a water source, leading to chemical precipitation.\( \newcommand\). Injecting fertilizers or soil amendments that contain calcium can cause calcium carbonate precipitation and using fertilizers that contain phosphate can cause calcium phosphate precipitation. In addition to the precipitation of natural chemicals, fertigation (the injection of fertilizer into irrigation water) can cause precipitates in the water that can clog emitters. To understand the formation of a solid in a chemical reaction, it is important to understand the key components that yield the precipitate.Precipitation reactions specifically occur when aqueous solutions of ionic solutes are mixed to produce a solid. Iron precipitate clogging is generally not so common a problem as lime (calcium carbonate) precipitate clogging, but where it does occur it is a difficult problem to mitigate. These types of chemical reactions are called precipitation reactions. Reactions similar to those that cause iron precipitation also cause manganese to precipitate. Iron bacteria in a well can also cause insoluble iron to form.
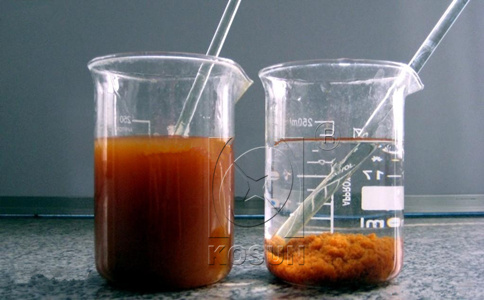
When you pump the water, the soluble iron can change to an insoluble form and, as a result, the iron precipitates out of the water. Iron exists naturally in groundwater as soluble iron. Increasing temperatures decrease the solubility of calcium carbonate, increasing the potential for precipitation. Schwankl Naturally occurring chemicals in groundwater that is used for irrigation can leave chemical precipitates in emitters, such as calcium carbonate (lime), iron and manganese compounds, and iron sulfide.Ĭalcium carbonate will precipitate when the bicarbonate concentration exceeds about 2 meq/l (milliequivalents per liter) and the pH is greater than 7.5.


 0 kommentar(er)
0 kommentar(er)
